The Art of Immune Warfare - The War
- S A

- Jun 1, 2020
- 11 min read
Updated: Jun 8, 2020
Now that we are locked and loaded, lets get the War started!

The Invasion
From the day we are born to the time we die, we humans are targets for attack by multitudes of other living organisms. From the air we breathe, to the soil we walk on, the water we drink to the building we live/work in; we are surrounded by forms of life that are potentially dangerous. These foreign organisms are divided into different groups on the basis of their size, biochemical characteristics, or manner in which they interact with us and can be categorized as bacteria, viruses, fungi, and parasites amongst other things.

Some bacteria like pneumococci, staphylococci and streptococci, all of which are often commensals (organisms living harmlessly on their hosts) in the upper respiratory tract but that can become virulent and cause serious conditions, such as pneumonia and septicemia. Pneumococci, on the other hand, often spread directly into the central nervous system, causing one of the common forms of meningitis.
Viruses can cause a simple cold sore to more serious conditions including death in some immune-compromised people. For example, when the measles virus enters the body, it multiplies for a week or two and then enters the bloodstream and spreads to every organ. Viruses like HSV-1 (Herpes Simplex virus) can cause infections of the eye, central nervous system, and the skin. Seasonal influenza viruses, for example, circulate globally every year, causing illness in tens of millions of people worldwide; an estimated 250,000 to nearly 600,000 people die from seasonal influenza each year. Some viruses “jump” to humans from an animal reservoir, such as bats, pigs, or primates; this occurs when a human is in close contact with an animal that carries the virus.
Parasites like whipworms, pinworms etc can target our large intestine or gastrointestinal tract causing a host of conditions like poor appetite, loss of weight, anemia, diarrhoea etc.
These pathogens can spread through a variety of ways.

The Attack - interplay of Innate and Adaptive Immune cells
Most microorganisms encountered in daily life are repelled before they cause detectable signs and symptoms of disease. These potential pathogens are quite diverse, and hence a nonspecific defense system is the first and foremost line of attack. Our innate immune system provides this kind of nonspecific protection through a number of defense mechanisms as we looked into in the previous blogs. Physical barriers provide the first line of defense, chemicals though not involved in defense but help in repelling and limiting the spread of the pathogens, complement proteins work together to lyse (break apart), harmful infectious organisms that do not have protective coats and finally the body brings in the big guns, the specialists, our adaptive immune response is enlisted to finish the pathogens off and make a record in form of memory cells. These cells hang out in our body, particularly in the lymph nodes, patrolling the fluid for a matching pathogen (with its matching antigen). Should these memory cells come across a match, they reactivate and attack in their corresponding ways. So our immune system knows exactly what needs to be done if similar pathogens decide to wage a war again.

Now lets look at the sequence of events that take place once a pathogen (or even an injury) enters our body and gets past our physical barriers. The following stages need not necessarily occur one after the other. Some of them happen in tandem.
Pathogen Recognition – Screening and Tracking
Upon a microbial infection, the body needs to be alerted to the presence of potential harmful pathogens. In order to detect pathogens such as bacteria and viruses the immune system (neutrophils, macrophages and dendritic cells) is equipped with receptors called pattern recognition receptors (PRRs) which sense the presence of a pathogen. These are predominantly expressed on immune cells.
These receptors recognize conserved molecular structures known as pathogen or damage-associated molecular patterns (PAMPs and DAMPs) that are found on microbes such as bacteria, viruses, parasites or fungi. These patterns are usually specific to the micro-organism (i.e. they are not present in the host and therefore are considered as “non-self”).

This can be thought off as another set of lock and key mechanism similar to Antigens and Antibodies. The key difference is PPRs are used for initial screening to recognize foreign cells (pathogens) and once these foreign cells are recognized and bound to, via PAMPs they are phagocytized (and also release chemicals which draw in more phagocytes) and small fragments (antigens) of these pathogens are displayed on the cell surfaces of the phagocytes (hence these are also called Antigen Presenting Cells or APCs) with the help of MHCs (Major Histomcompatibility Complex - In this case II for foreign antigen. MHC I is for self antigens) molecules. In humans these are called Human Leukocytic Anitgens (HLA). This is where our adaptive immune system come into play where they have a corresponding lock which complements these antigens thereby binding to them and initiating the adaptive immune response.
The presenting of invader's antigens on the HLA molecules of the APCs is one of the main bridges between the innate and adaptive immune response.

Upon binding of PRRs with PAMPs (pathogen recognition), they induce various cellular responses including the transcription of several genes that ultimately will result in the elimination of the pathogen. Immune cells release cytokines to tell other cells to start fighting back. The signals derived from the engagement of PRRs on the immune cells activate microbicidal (something that destroys microbes) and pro-inflammatory responses required to eliminate or, at least, to contain infectious agents.
Once a pathogen is detected, the immune system must also track whether it is replicating intracellularly (inside the cell, as with most viruses and some bacteria) or extracellularly (outside of the cell, as with other bacteria, but not viruses). The innate immune system will respond accordingly by identifying the extracellular pathogen and/or by identifying host cells that have already been infected. The binding of PRRs with PAMPs triggers the release of cytokines, which signal that a pathogen is present and needs to be destroyed along with any infected cells.
Key Players: Neutrophils, Macrophages and Dendritic cells
Function: Phagoctyize as many pathogens as possible and signal to bring in extra cavalry to finish the rest who mange to escape!
Inflammatory response:
The release of damaged cellular contents stimulates the inflammatory response. The inflammatory reaction brings in phagocytic cells into the site to destroy the pathogen and remove it and debris from the site, but also helps to isolate the site, limiting the spread of the pathogen. Inflammation can be Acute or Chronic. Acute inflammation is a short-term inflammatory response to an insult to the body. If the cause of the inflammation is not resolved, however, it can lead to chronic inflammation, which is associated with major tissue destruction and fibrosis.

Inflammatory response is carried out in four stages:
Stage I: Tissue damage and Release of Histamine
Tissue damage caused by toxin, microorganism or mechanical, mast cells (localised) and basophils (circulating) release a chemical called histamine.
Stage II: Vasodilation
Histamine acts on surrounding blood capillaries and causes vasodilation. When vasodilation occurs. Increased blood flow causes redness and heat
Stage III: Increased permeability
At the same time histamine increases the permeability of blood capillaries leading to leakage of fluid from blood capillaries. This results in fluid accumulation causing swelling and tenderness. Capillary permeability improves the recruitment of leukocytes to the region.
Stage IV: Extravasation
Within few hours, Neutrophil migrates to the site of tissue damage by the process of chemotaxis (movement of a cell, immune cell in this case in response to a chemical signal) and passes through capillaries wall and enter into tissue space by the process called extravasation (to force something out of a vessel).

Key Players: Mast Cells, Basophils and Neutrophils – Histamine, Prostaglandins
Function: Induce inflammation to contain the pathogens
Phagocytosis - Engulf the suckers!!
Phagocytosis is the simplest of immune response mechanisms and probably older than multicellular life. The invader or foreign matter is just surrounded and digested by macrophages, dendritic cells and neutorphics; which will engulf the pathogen and break them apart. When macrophages engulf exogenous pathogens, they digest them within lysosomes to release antigenic fragments. These fragments are presented on special surface receptors (MHC class II) that denote the material as being foreign. Phagocytosis is probably the body’s most frequently used mechanism.

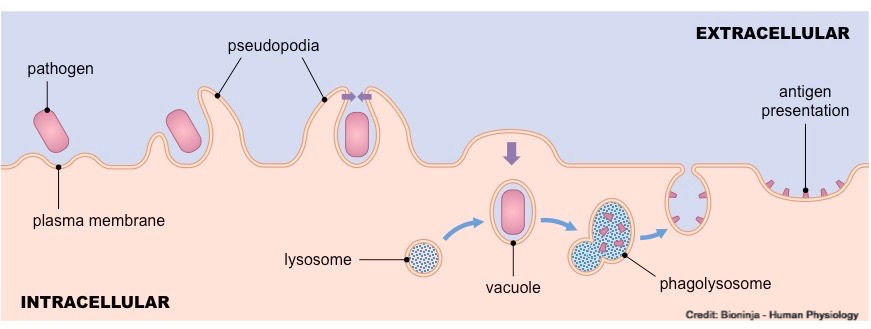
Neutrophils, Dendritic Cells, Eosinophils (limited role) and Macrophages
Function: Engulf and kill the pathogens
Degranulation – Kill the suckers!!
Once the pathogens are engulfed, they are killed by the release of certain chemicals.Various types of WBCs have granules in their cytoplasm. Granules aren’t chemicals but little packets of chemicals, such as histamine, cellular toxins, enzymes, and other proteins. Through degranulation, WBCs move the granules out of the cell, releasing the chemicals into the interstitial space (via exocytosis). After they’ve been absorbed into the interstitial fluid, these chemicals carry out a range of specialized immune functions. Some destroy invaders directly. Some regulate immune system processes.

Because neutrophils are the most numerous of the WBCs, their granulocytic properties are very important in the immune response, especially to bacterial infection. Mast cells are granular cells that play a key role in the inflammatory process. When activated, a mast cell rapidly releases the contents of its granules and various hormonal mediators into the extracellular space. They’re involved in allergic reactions, anaphylaxis, and autoimmunity.
Neutrophils: (Lysozymes, Defensins, Hydrogen Peroxide) - These are phagocytic and granulocytic
Eosinophils: Eosinophil peroxidase, cytokines, chemokines
Basophils: histamine and heparin
Mast cells: histamine, interleukins and heparin
Natural Killer Cells: Perforin - a protein that forms pores in the membranes of infected cells Granzymes - a protein-digesting enzyme that enters the cell via the perforin pores and triggers apoptosis intracellularly.
Cellular Signalling - Raise the Alarm
Upon recognition of an invasion by a pathogen, phagocytes will bind using its PRR to the PAMP of the pathogen. They will then engulf the pathogen and partially degrade it, and export fragments (antigens) of the microbe to the cell surface, where they are presented in association with MHC II molecules on its surface. It also triggers the release of cytokines, which signal that a pathogen is present and needs to be destroyed along with any infected cells. A receptor on the surface of the helper T Cell then binds to the MHC-Antigen complex.
Helper T Cells can be divided into subtypes TH1 and TH2 cells and they have significantly different chemistry and function. The main role of the TH1 cells is to stimulate cell-mediated responses (those involving cytotoxic T cells and macrophages), while TH2 cells primarily assist in stimulating B cells to make antibodies. Once the initial steps of activation have occurred, helper T cells synthesize other proteins, such as signaling proteins and the cell-surface receptors to which the signaling proteins bind. These signaling molecules play a critical role not only in activating the particular helper T cell but also in determining the ultimate functional role and final differentiation state of that cell.

TH1 cells primarily produce the cytokines gamma interferon, TNF-beta, and interleukin-2, while
TH2 cells mainly synthesize the interleukins IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13.
Once the helper T Cell binds to a phagocyte, it also needs to be stimulated either by a cytokine or through a co-stimulatory reaction between the signaling protein, B7, found on the surface of the antigen-presenting cell, and the receptor protein, CD28, on the surface of the helper T cell. It needs both the first signal (binding to MHC-antigen complex) and one of the two second signals for Helper T Cell to be activated!!
The activated Helper T Cell (TH2) releases cytokines, which will in-turn stimulate B Cells. Cytokines stimulate the infected cells and those nearby to produce proteins that prevent the virus from replicating within them. Cytokines released by TH1, In particular, gamma interferon greatly increases the ability of macrophages to kill ingested microbes; this can tip the balance against microbes that otherwise resist killing. Gamma interferon also stimulates natural killer cells.

In addition to being released by phagocytes after PAMP recognition, cytokines (Interleukins) are also released by the infected cells which bind to nearby uninfected cells, inducing those cells to release cytokines, resulting in a cytokine burst. Interleukins are released by phagocytes play a key role in activating Helper T and B Cells. A second class of cytokines (interferons), which are released by infected cells as a warning to nearby uninfected cells. Interferons work by signalling neighbouring uninfected cells to destroy RNA (often a very important biomolecule for viruses) and reduce protein synthesis.
Cytokines also send feedback to cells of the nervous system to bring about the overall symptoms of feeling sick, which include lethargy, muscle pain, and nausea. These effects may have evolved because the symptoms encourage the individual to rest, preventing them from spreading the infection to others. Cytokines also increase the core body temperature, causing a fever, which causes the liver to withhold iron from the blood. Without iron, certain pathogens (such as some bacteria) are unable to replicate; this is called nutritional immunity.

Key Players: Macrophages, Dendritic and Helper T Cells - Cytokines
Function: Cellular Signalling
Apoptosis – Initiate suicide mission
Virus infected cells can also be destroyed non-specifically by Natural Killer and Cytotoxic T cells, which respond to interferons released by the infected cell. Cytotoxic T cells (granular) can kill their target cells either through the use of pore-forming molecules, such as perforins (which literally put holes in the cells) and various components of cytoplasmic granules, or non granular cytotoxic T cells by triggering a series of events with the target cell that activate cell death program, a process called apoptosis. Interferons signal neighbouring infected cells to undergo apoptosis (programmed cell death). So the target cells pretty much sacrifice themselves for the greater good by committing suicide, thereby destroying the virus within the cell as well.

Key Players: Natural Killer and Cytotoxic T cells - Cytokines
Antibodies
Cytokines secreted by cytotoxic T Cells stimulate B cells. These B Cells are segregated into plasma cells which produce large quantities of specific antibodies (lock) that are a perfect match for the pathogen’s antigen (Key). B Cells further divides into Plasma cells, which are short-lived and secrete high numbers of antibodies that are specific to a particular antigen. Plasma cells will secrete ~ 2,000 antibody molecules per second into the bloodstream for roughly 4 to 5 days. A small percentage of the B Cells will form memory cells, which keeps a record of the encountered pathogen and forms part of our long term immunity.

Memory cells are long living and will survive in the body for many years, producing low levels of circulating antibodies. If a second infection with the same pathogen occurs, memory cells will react more vigorously to produce antibodies faster. As antibodies are produced faster, the pathogen cannot reproduce in sufficient amounts to cause disease symptoms. Because the pathogen exposure no longer causes the disease to occur, the individual is said to be immune. However memory cells have a shelf life as well and we lose some of this immunity over the course of time if we are not exposed to the pathogens again.
Usually there is delay between the initial exposure to a pathogen and the production of large quantities of antibodies. If pathogens can reproduce rapidly during this delay period, they can impede normal body functioning and cause illness/disease.
Antibodies aid in the destruction of pathogens by a number of different mechanisms:
• Precipitation – Soluble pathogens become insoluble and precipitate
• Agglutination – Cellular pathogens become clumped for easier removal
• Neutralisation – Antibodies may occlude (block) pathogenic regions (e.g. exotoxins)
• Inflammation – Antibodies may trigger an inflammatory response within the body
• Complement activation – Complement proteins perforate membranes (cell lysis)

Key Players: B Cells - Plasma and Memory Cells
Our immune system is constantly working hard to keep us safe from the pathogens. All three defense and attack systems like the physical barriers, innate and adaptive immune systems work together to keep pathogens out. Whereas barrier defenses are the body’s first line of physical defense against pathogens, innate immune responses are the first line of physiological defense. Innate responses occur rapidly, but with less specificity and effectiveness than the adaptive immune response. Innate immune response does not stop when the adaptive immune response is developed. In fact, both can cooperate and one can influence the other in their responses against pathogens and keep us safe and build our immunity through memory cells.

This is how our immune system manages to win the war against various pathogens out there. So how do we keep it optimal? Is there really such a thing as boosting one's immunity. How does Covid-19 fit into all of this. That's coming up next





Comments